Radium
88
Ra
Group
2
Period
7
Block
s
Protons
Electrons
Neutrons
88
88
138
General Properties
Atomic Number
88
Atomic Weight
[226]
Mass Number
226
Category
Alkaline earth metals
Color
Silver
Radioactive
Yes
From the Latin word radius meaning ray
Crystal Structure
Body Centered Cubic
History
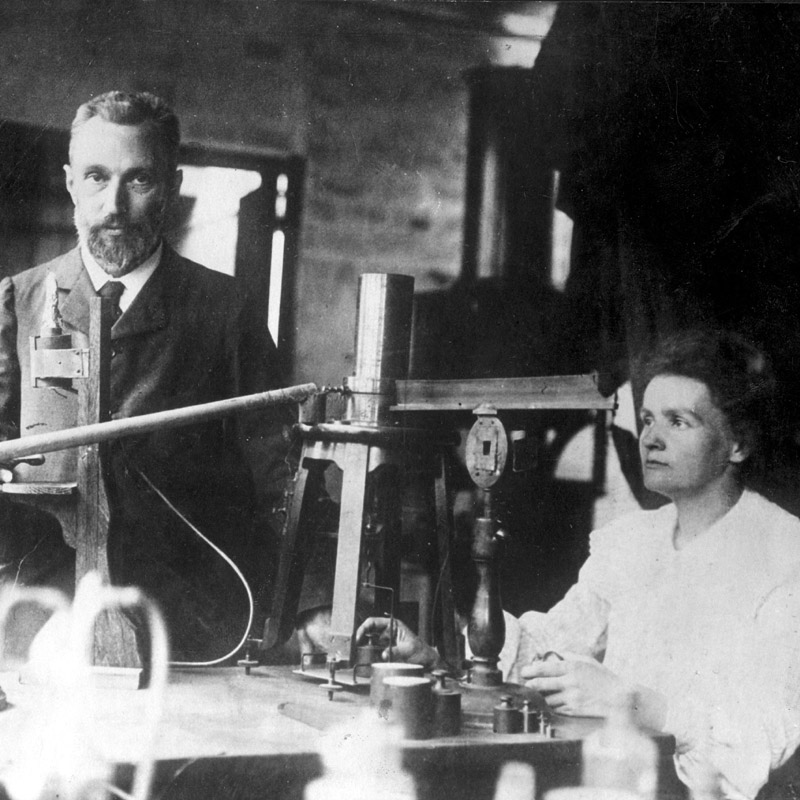
Radium was discovered by Marie Curie and Pierre Curie in 1898.
They extracted the radium compound from a uraninite sample.
Radium was isolated in its metallic state by Marie Curie and André-Louis Debierne in 1910 through the electrolysis of radium chloride by using a mercury cathode and distilling in an atmosphere of hydrogen gas.
They extracted the radium compound from a uraninite sample.
Radium was isolated in its metallic state by Marie Curie and André-Louis Debierne in 1910 through the electrolysis of radium chloride by using a mercury cathode and distilling in an atmosphere of hydrogen gas.
Electrons per shell
2, 8, 18, 32, 18, 8, 2
Electron Configuration
[Rn] 7s2
Radium imparts a carmine red color to a flame
Physical Properties
Phase
Solid
Density
5.5 g/cm3
Melting Point
973.15 K | 700 °C | 1292 °F
Boiling Point
2010.15 K | 1737 °C | 3158.6 °F
Heat of Fusion
8 kJ/mol
Heat of Vaporization
125 kJ/mol
Specific Heat Capacity
-
Abundance in Earth's crust
9.9×10-12%
Abundance in Universe
n/a
CAS Number
7440-14-4
PubChem CID Number
6328144
Atomic Properties
Atomic Radius
-
Covalent Radius
221 pm
Electronegativity
0.9 (Pauling scale)
Ionization Potential
5.2784 eV
Atomic Volume
45.20 cm3/mol
Thermal Conductivity
0.186 W/cm·K
Oxidation States
2
Applications
Radium was formerly used in self-luminous paints for watches, nuclear panels, aircraft switches, clocks, and instrument dials.
Radium chloride was used in medicine to produce radon gas which in turn was used as a cancer treatment.
The isotope 223Ra is currently under investigation for use in medicine as a cancer treatment of bone metastasis.
Radium chloride was used in medicine to produce radon gas which in turn was used as a cancer treatment.
The isotope 223Ra is currently under investigation for use in medicine as a cancer treatment of bone metastasis.
Radium is highly radioactive and carcinogenic
Isotopes
Stable Isotopes
-Unstable Isotopes
202Ra, 203Ra, 204Ra, 205Ra, 206Ra, 207Ra, 208Ra, 209Ra, 210Ra, 211Ra, 212Ra, 213Ra, 214Ra, 215Ra, 216Ra, 217Ra, 218Ra, 219Ra, 220Ra, 221Ra, 222Ra, 223Ra, 224Ra, 225Ra, 226Ra, 227Ra, 228Ra, 229Ra, 230Ra, 231Ra, 232Ra, 233Ra, 234Ra